Medical Device Usability by Design™
Human Factors is all about understanding the interaction between products, the people who use them, the tasks performed with them, and the environments where they are used. The proper application of Human Factors principles throughout the development process results in better, safer, more usable medical devices.
A User Centered Design (UCD) methodology is the cornerstone of the FDA's Human Factors Guidance for medical device design. It's a proven way to reduce risk, enhance safety for both patient and clinician, and expedite regulatory approval.
Done correctly, UCD also increases the likelihood of market success -- because the product was designed with meaningful input from the clinicians, patients, or caregivers who will use it.
Usability is the key to clinical adoption and patient compliance.
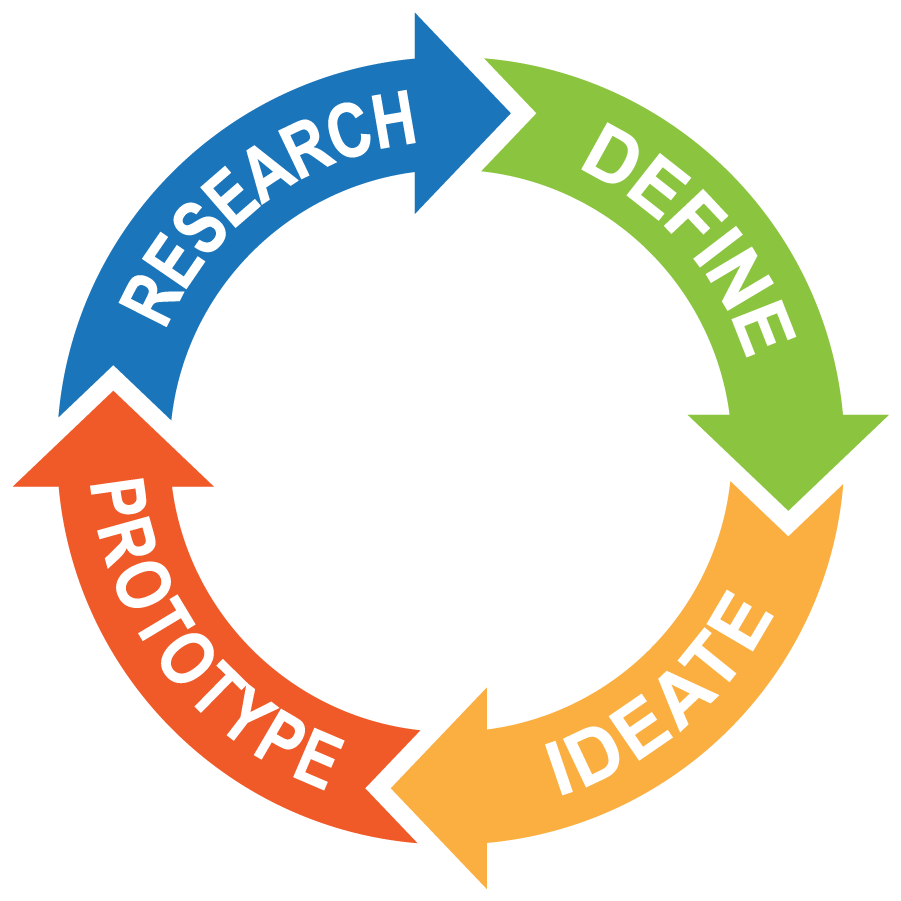
At a Glance
Service Offerings
No matter what stage of the product development cycle you’re currently in, I can give you confidence that your product’s features will resonate with users. I will work with you to create and execute a plan which may include:
Contextual Inquiry
Planning, conducting, and documenting Ethographic Research (a.k.a., Voice of the Customer) in the intended use environment, to better understand user needs, workflows with predicate devices, and opportunities for improvement
Requirements
Identifying and documenting the User Needs that will serve as the basis for Product Specifications, to drive the conceptualization efforts of Industrial Designers, Interaction Designers, and Engineers
Sketch Ideation
Exploring system architectures and brainstorming individual feature embodiments that represent a wide range of possible solutions

Form Study Models
Creating hand-sculpted or 3D-printed models, to explore concept alternatives and evaluate proposed grip architectures

Preliminary CAD
Creating 3D CAD models of research stimuli, in just enough fidelity to enable users to simulate the use of proposed features

Interaction Design
Creating Interaction Models and Wireframes to explore concept alternatives for digital displays and workflows
UX Models
Building low-fidelity User Experience (UX) Models to explore and evaluate proposed features and workflows

Formative Study
Designing, conducting, and documenting a simulated-use study with representative users (often using low-fidelity, non-functional models) to evaluate concept alternatives, helping detect usability problems while there is still time to design them out

Task Analysis
Performing Use FMEAs and/or Task Analysis to identify Use Scenarios and Critical Tasks that need to be tested as part of a Summative Study

Summative Study
Designing, conducting, and documenting a simulated-use study to compile data demonstrating that appropriately trained users can successfully complete the Critical Tasks

Validation Testing
Designing, conducting, and documenting Validation Testing as part of a Quality Management System, to provide objective evidence that first article product samples conform to user needs and intended uses

HF Documentation
Authoring elements of the Usability Engineering File (for 62366-1:2015 compliance) and HFE/UE Report (to satisfy the FDA Human Factors guidance) throughout the product development process, in support of regulatory submissions

About Me
My name is Craig Conner. I'm a Human Factors Engineer and Product Designer.
My focus on User Centered Design draws from a unique educational background in Design Engineering, Human Factors, and the Fine Arts.
As an undergrad studying Mechanical Engineering at the University of Notre Dame, I was drawn to the field of Product Design. While in graduate school at the University of Wisconsin–Madison studying Product Design Engineering, I found my true passion in the Human Factors curriculum of the Industrial Engineering department.
During both degree programs, I continued my life-long study of art, taking studio art courses in Industrial Design and Sculpture, to more formally study how form follows function.
Throughout my career, I've successfully blended research acumen with creative problem solving – always bringing an understanding of human capabilities and limitations to the forefront. I enjoy playing in the creative sandbox alongside Industrial Designers, Mechanical Engineers, and Digital Interaction Designers, especially in the fuzzy front end of innovation. I help ensure that the products that emerge are grounded in usability, feasibility, and desirability.
Commercial Success
A sample of medical devices from my portfolio that are currently in the market

G6 Applicator by Dexcom
Handheld device used by patients to place the body-worn component of a continuous glucose monitor
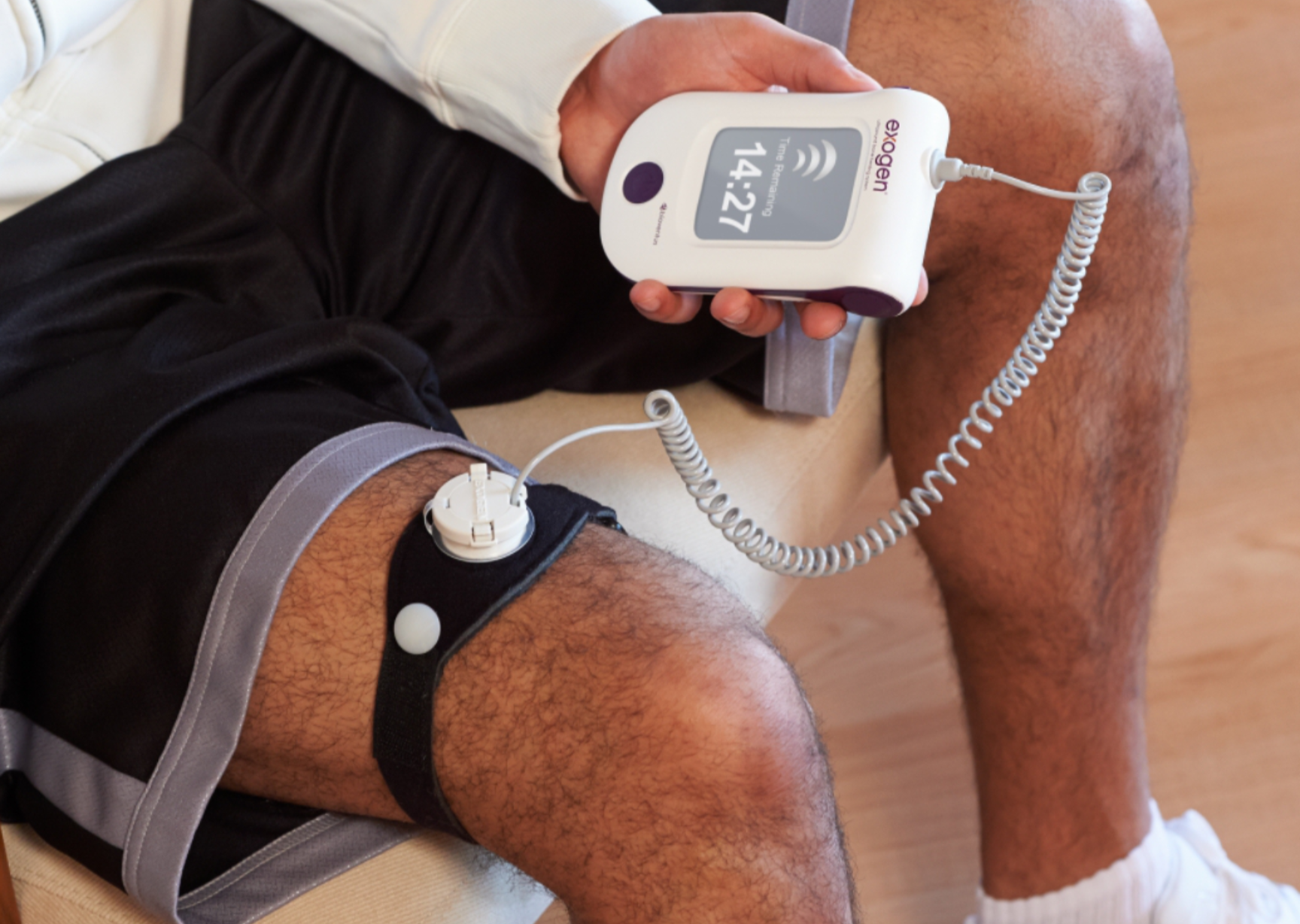
Exogen by Bioventus
Ultrasound bone growth stimulator for healing fresh and nonunion fractures
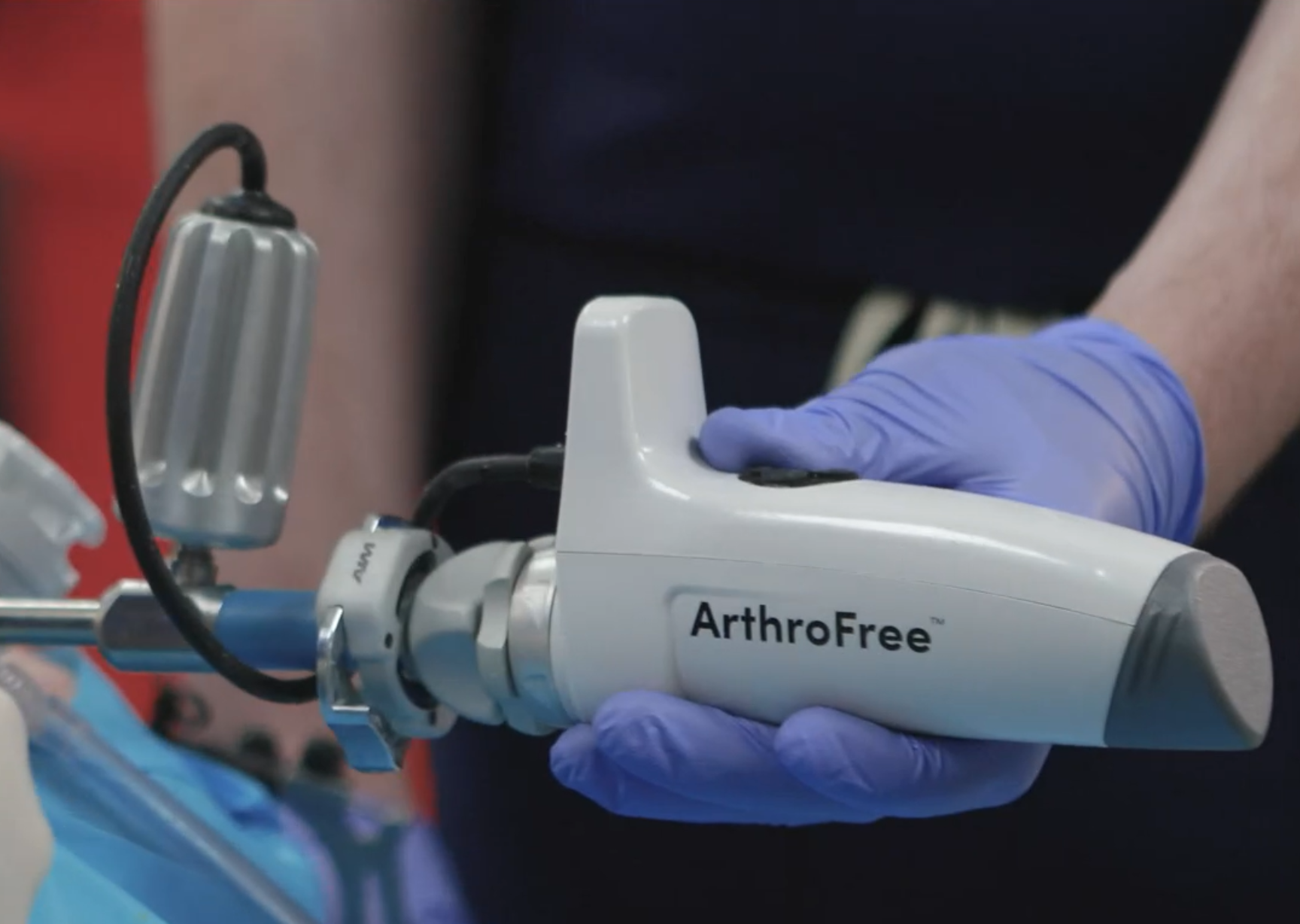
ArthroFree by Lazurite
Wireless arthroscope for exploratory and reconstructive surgery, removing the hazards of conventional fiber optic and power cables

Otoscan by Natus Medical
Headset enabling a non-invasive 3D digital laser scanning of the ear canal, for custom hearing aids

Tru-Fit Overbed Table Storage by Stryker
Integrated storage attachment for use by both patients in Med/Surg unit and clinicians in the ICU
https://www.stryker.com/us/en/acute-care/products/tru-fit.html
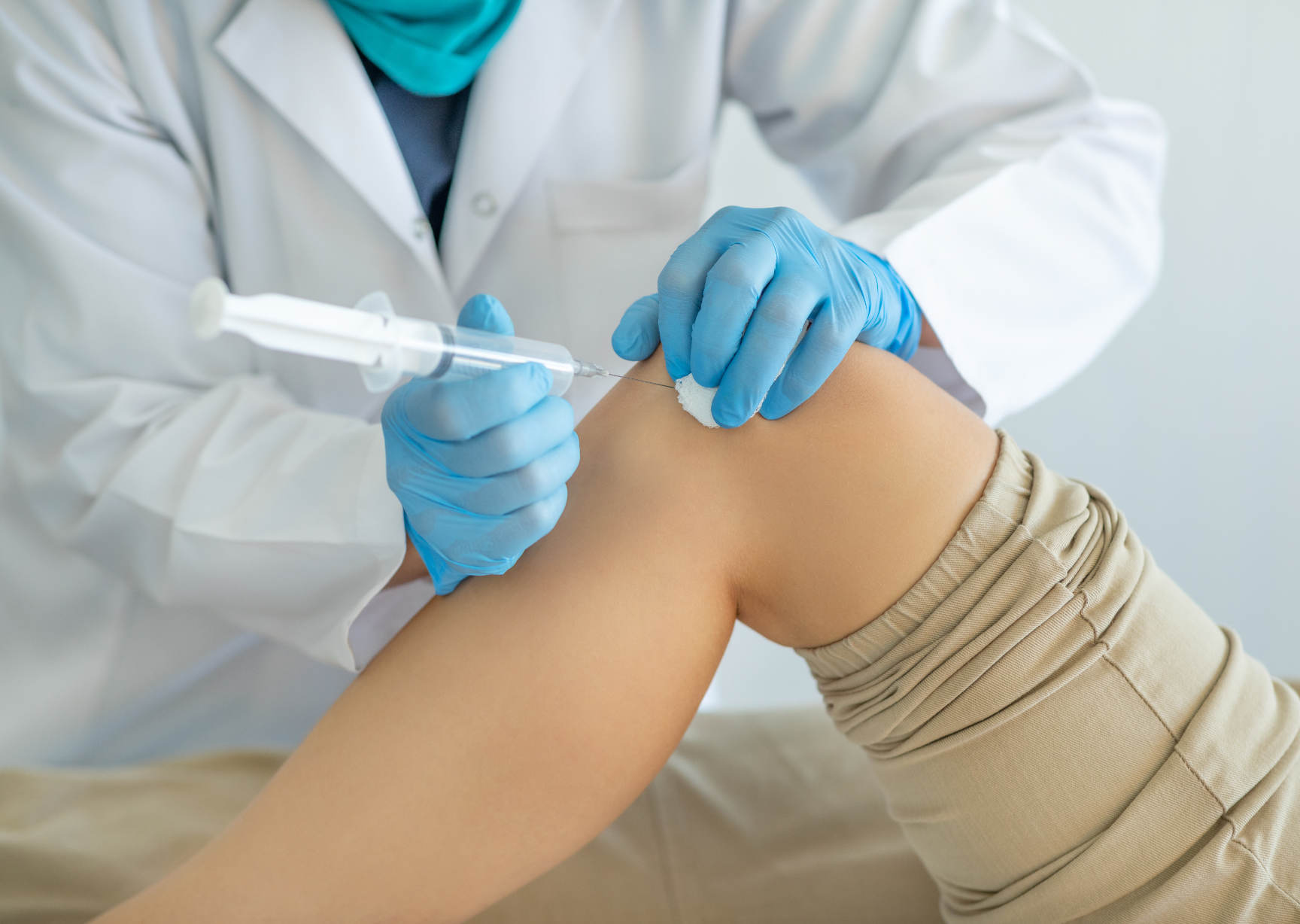
Supartz by Bioventus
Injection of hyaluronic acid to relieve knee pain from osteoarthritis
